NAD+ (Nicotinamide Adenine Dinucleotide)
- Regular price
-
$69.00 USD - Regular price
-
$79.99 USD - Sale price
-
$69.00 USD
Nicotinamide Adenine Dinucleotide (NAD+) is a coenzyme that is present in each living cell in the body. It is produced from the breakdown of nicotinamide riboside (niagen), an alternative form of vitamin B3 (niacin). NAD+ plays an integral role in energy production and regulation of vital cellular processes such as DNA repair, strengthening cells’ defense systems, conversion of food into a usable form of energy, and regulation of circadian rhythm.
How NAD+ Works
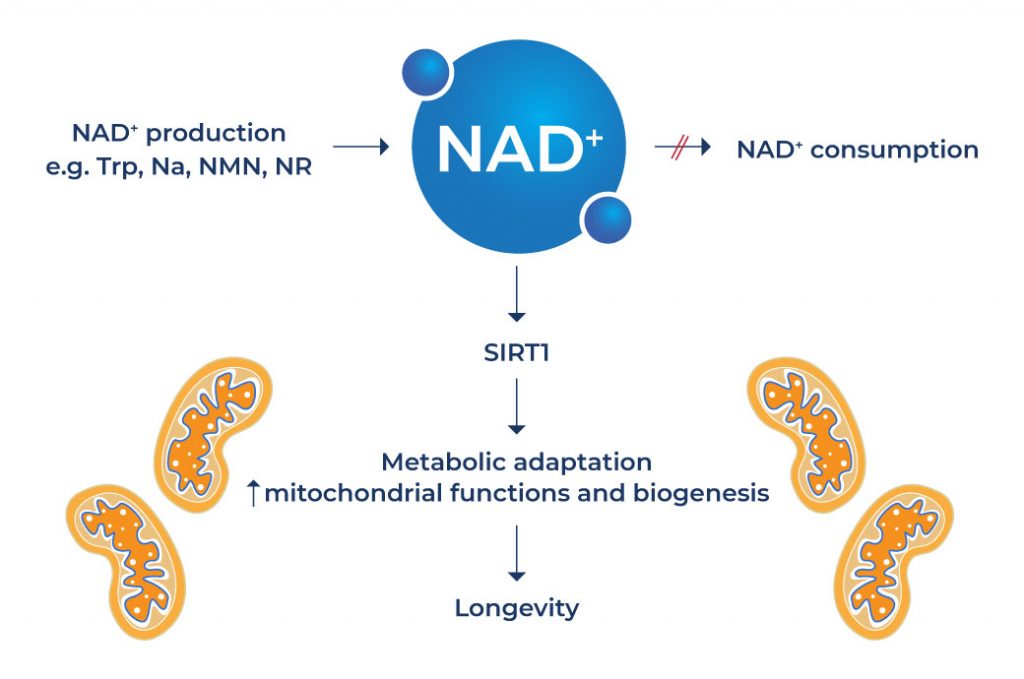
NAD+ converts nutrients into adenosine triphosphate, a compound that provides energy to living cells. Aside from this important function, it works together with various forms of proteins to carry out a wide array of biological processes such as DNA repair, calcium signaling, maintenance of cell energy and chromosomal integrity, and gene expression.
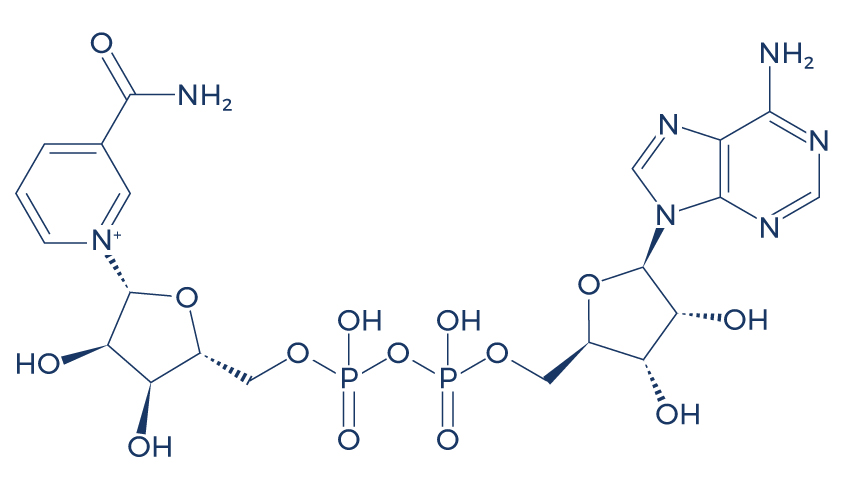
Chemical Structure of NAD+
Research on NAD+
A. Extends Lifespan
Aging is associated with progressive restriction in the length of telomeres, which are located at chromosome ends. They play an important role in the preservation of chromosome stability. Studies have shown that individuals with longer telomeres have a longer subsequent lifespan. [1-2] Increasing sirtuin activity is known to stabilize telomeres and attenuate age-related telomere shortening. [3] Since NAD+ activates SIRT1, it can help achieve chromosome stability and longer telomeres – all of these mechanisms can increase longevity.
The natural process of aging is associated with a decline in the quality and activity of the “powerhouse of the cell” known as the mitochondria. As the name implies, the mitochondria produce the energy needed to power the cell’s biochemical reactions – everything from the transmission of signals, digestion, muscle function, and other essential bodily processes. Mitochondrial function is a determinant of lifespan and studies show that mitochondrial dysfunction can shorten lifespan. [4-5]
Another mechanism that can help extend lifespan is through the activation of SIRT1 function in the nucleus of cells. Evidence suggests that boosting NAD+ levels through NAD+ supplementation can dramatically ameliorate mitochondrial dysfunction via activation of sirtuin 1 (SIRT1). [6] SIRT1 is an enzyme that plays an integral role in the regulation of proteins involved in cellular metabolism and processes associated with longevity, inflammation, and stress. SIRT1 activation by NAD+ can increase longevity by promoting mitochondrial biogenesis, a cellular process that involves the production of new mitochondria. [7]
A convincing number of evidence suggests that NAD+ can extend lifespan:
- The administration of the NAD+ precursor nicotinamide riboside extended the lifespan of mice without calorie restriction. [8]
- In mice with ataxia-telangiectasia (A-T), a rare disease characterized by progressive neurodegeneration that affects movements and speech, NAD+ replenishment improved lifespan and health span by stimulating neuronal DNA repair and improving mitochondrial quality. [9]
- In a mouse model of amyotrophic lateral sclerosis (ALS), a medical condition characterized by progressive degeneration of motor neurons, supplementation with a bioavailable NAD+ precursor (nicotinamide riboside, NR) delayed the degeneration of motor neurons, decreased spinal inflammation, and improved survival rate. [10]
- In a mouse model of muscular dystrophy, a condition characterized by gradual weakening of the muscles, treatment with the NAD+ precursor nicotinamide riboside (NR) delayed muscle breakdown and increased lifespan. [11]
- In a mouse model of chronological aging, the administration of CD38 inhibitor 78c increased NAD+ levels which in turn improved exercise performance, endurance, and metabolic function and increased lifespan. [12]
- In Caenorhabditis elegans, a type of worm, NAD+ increased lifespan through the activation of sirtuin (SIR-2). [13-14]
- In Saccharomyces cerevisiae, a species of yeast, NAD+ extended the lifespan and ameliorated aging-related conditions. [15]
B. Anti-Aging Benefits
While a decline in the function of the mitochondria has been linked with normal aging, this is also associated with a wide array of age-related medical conditions. Evidence suggests that mitochondrial aging contributes to cellular senescence (also known as biological aging), increased inflammation, decreased stem cell activity, reduced healing rate, and a decline in tissue and organ function. [16] Interestingly, NAD+ can reverse age-related mitochondrial dysfunction via SIRT1 activation.
New research found that some of the age-related changes in the structures of the mitochondria can be reversed through NAD+ supplementation. [17] In this study, the administration of NMN (nicotinamide mononucleotide), a molecule that boosts NAD+ levels, via injections in elderly mice reversed age-related mitochondrial deterioration. It was observed that declining NAD+ levels were associated with interruptions in the normal signaling between the cell’s nucleus and mitochondria. Interestingly, raising NAD+ levels restored the communication between these cellular structures.
During the normal process of aging, DNA damage occurs continuously on a massive scale. The age-related DNA damage contributes to cell death and a decline in the function of neurons or nerve cells. This creates a domino effect, causing a gradual decline in a number of bodily functions. NAD+ can mitigate these effects since it is connected to the DNA repair precursor poly adenosine diphosphate-ribose polymerase 1 (PARP 1). PARP 1 consumes NAD+ in the presence of DNA damage in order to promote cell survival. This in turn can help slow down the effects of aging.
Preclinical evidence also shows that boosting NAD+ levels can help mitigate age-related functional decline:
- In elderly mice, treatment with the NAD+ booster nicotinamide mononucleotide (NMN) improved blood flow and increased endurance via the promotion of SIRT1-dependent increases in capillary density. [18]
- In mice with progressive weakness and loss of muscle mass, NAD+ repletion improved muscle and mitochondrial function. [19]
- In mice, the administration of the NAD+ booster nicotinamide mononucleotide prevented age-related weight gain and improved physical activity, energy metabolism, lipid profiles, and insulin sensitivity. [20]
- In mouse models of retinal dysfunction, NAD+ deficiency caused metabolic dysfunction in the cells of the retina of the eye, suggesting that restoring NAD+ to youthful levels can help age-related vision loss. [21]
- In mice with mitochondrial dysfunction, oral administration of nicotinamide riboside (NR), a NAD+ precursor, increased mitochondrial numbers and prevented muscle abnormalities. [22]
- In mice, the administration of nicotinamide riboside (NR) prevented noise-induced hearing loss and degeneration of ear cells, suggesting that increasing NAD+ levels can help age-related hearing loss. [23]
- In mice fed with a high-fat diet, the administration of the NAD+ booster nicotinamide mononucleotide prevented diet- and age-induced diabetes by enhancing insulin sensitivity. [24]
C. Increases Energy Levels
NAD+ plays an integral role in energy production and regulation of vital cellular processes. This includes the conversion of food into a usable form of energy called adenosine triphosphate.
Studies suggest that increased ATP production caused by NAD+ may help boost energy levels and reduce fatigue:
- In old mice, supplementation with a NAD+ precursor improved ovarian mitochondrial energy metabolism. [25]
- In obese mice, chronic NAD+ supplementation (1 mg/kg/day for the last 4 weeks) significantly attenuated weight gain and improved diurnal locomotor activity patterns. [26]
- In patients with chronic fatigue syndrome, NAD+ supplementation decreased anxiety and improved heart rate. [27]
- A study reported that NAD+ may be a promising intervention to overcome symptoms of fatigue and to improve the quality of life of patients with chronic fatigue syndrome. [28]
- The use of coenzyme Q10 (CoQ10) plus NAD+ supplementation reduced perceived cognitive fatigue and improved the health-related quality of life in patients with chronic fatigue syndrome. [29-31]
- In trained and untrained subjects, NAD+ supplementation was associated with improved exercise endurance. [32]
- 7. NAD+ treatment was found to be more effective than conventional therapy for chronic fatigue syndrome. [33]
D. Promotes Weight Loss
The ability of NAD+ to induce weight loss can be attributed to its energy-boosting mechanisms. With increased energy expenditure, the body will not store additional fat. Instead, the metabolism is increased, resulting in weight loss.
There’s a great deal of evidence supporting the fat-burning effects of NAD+:
- In obese female mice, NAD+ injections reversed glucose intolerance induced by obesity and improved exercise capacity. [34]
- In healthy obese participants, NAD+ supplementation reduced weight by 17.1%. [35]
- In mammalian cells and mouse tissues, NAD+ protected against oxidative stress and high-fat diet-induced metabolic abnormalities. [36]
- In mice, supplementation with NAD+ at 400 mg/kg/day reduced abdominal visceral fat deposition. [37]
- In a study of twins, lower NAD+ levels were associated with acquired obesity. [38]
- A cell study found that NAD+ can promote weight loss by reducing the number of adipocytes (fat cells). [39]
- Several studies have revealed that decreased NAD+ levels in cells were associated with higher fat mass tissues in the skeletal muscles, liver, and brain. [40-43]
- In mice, NAD+ protected against obesity by promoting whole-body energy homeostasis. [44]
- In mice, long-term administration of NAD+ reduced age-associated body weight gain. [45]
- NAD+ supplementation in mice ameliorated maternal obesity. [46]
- A cell study found that NAD+ normalized mitochondrial function and whole-body metabolism. [47]
- In mice fed with a high-fat diet, NAD+ protected against high-fat diet-induced metabolic abnormalities. [48]
E. Increases Muscle Mass and Strength
The anti-aging effects of NAD+ can also help attenuate the age-related decline in muscle mass and strength. Evidence suggests that NAD+ reverses detrimental age-associated changes in muscle by boosting ATP production, increasing mitochondrial function, and reducing inflammation. [49]
Studies show that NAD+ can help combat loss of muscle mass and strength associated with aging and musculoskeletal disease:
- In mice with progressive weakness and loss of muscle mass, NAD+ repletion improved muscle and mitochondrial function. [19]
- In mice with mitochondrial dysfunction, oral administration of nicotinamide riboside (NR), a NAD+ precursor, increased mitochondrial numbers and prevented muscle abnormalities. [22]
- In older men, NAD+ supplementation increased muscle mass and reduced circulating inflammatory cytokines. [50]
- In old mice, restoration of NAD+ levels to normal reversed skeletal muscle aging. [51]
- A 2018 study reported that NAD+ is essential in skeletal muscle development and regeneration. [52]
- In healthy obese men and women, administration of NAD+ at 1g per day for 6 weeks improved skeletal muscle composition. [53]
- A study found that aerobic and resistance exercise training can reverse age-related muscle loss by increasing NAD+ levels. [54]
- In a mouse model of Duchene’s muscular dystrophy (DMD), NAD+ supplementation improved muscle function. [55]
F. Improves Cognitive Function
A decline in NAD+ levels is associated with a number of neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease, and other brain disorders that cause cognitive dysfunction. [11] Interestingly, NAD+ has neuroprotective effects and the ability to decrease the production of reactive oxygen species (ROS), which are linked to a wide array of medical maladies including neurodegenerative diseases.
NAD+ can also help prevent cell death by regulating the activity of polyadenosine diphosphate-ribose polymerase 1 (PARP1). [56] PARP1 activity is usually increased in the brain of patients with Alzheimer’s disease and other neurodegenerative disease and is associated with increased deposition of abnormal protein structures in the brain. [57] Basically, NAD+ regulates PARP1 activity so that it will not kill too many cells before they can be repaired.
In addition, NAD+ plays important roles in a wide array of biological processes in the brain such as transmission of nerve signals, learning, and memory. [58] NAD+ helps increase the levels of brain chemicals called neurotransmitters, such as dopamine, serotonin, and norepinephrine, which are involved in a number of cognitive functions including memory, motivation, attention, mood, and emotions.
A number of strong scientific evidence suggests that NAD+ can help improve cognitive health:
- In a cell study, researchers found that cells treated with NAD+ were more resistant to stress. [59]
- According to a rat study, NAD+ helps protect the brain against oxidative stress. [60]
- A rat udy found that NAD+ can significantly decrease brain injury. [61]
- In a rat study, researchers found that NAD+ is also essential for altering genes that accelerate aging. [62]
- According to a rat study, NAD+ can slow or even reverse the progression of age-related brain diseases. [63]
- Studies found that decreased amounts of NAD+ in the cells accelerate the aging process of the brain. [64-67]
- A rat study found that NAD+ helps the brain function at optimal levels. [68-72]
- Numerous studies suggest that insufficient amounts of NAD+ result in cell breakdown, which in turn accelerates the aging process and causes mitochondrial dysfunction in the brain. [73-77]
- Several studies found that NAD+ is important for the continued production of energy (ATP) by the mitochondria in the brain. [78-81]
- Studies found that NAD+ helps maintain a healthy neurological system and protects against various neurological diseases. [82-84]
- A study found that NAD+ is an essential coenzyme needed for brain function. [85]
- Cell studies found that NAD+ can help improve the functions of the neurons in the brain. [86-88]
- In a mouse model of Alzheimer’s disease, NAD+ supplementation significantly normalized nerve cell inflammation, synaptic transmission, and DNA damage as well as improved learning, memory, and motor function. [89-95]
- A study found that NAD+ may help improve cognitive function through its anti-inflammatory effects. [96]
G. Fights Cancer
Mitochondrial respiration malfunction and increased glucose uptake are usually observed in cancer cells. Interestingly, increasing NAD+ levels has been shown to boost mitochondrial respiration and reduce glucose (blood sugar) uptake. [97] By counteracting these processes, NAD+ can help prevent the growth of cancer cells.
Increased NAD+ levels can also help boost the activity of SIRT1 and SIRT6, both of which inhibit the growth and spread of tumors via alteration of beta-catenin signaling and reduction of glucose uptake. [98-99]
Studies suggest that NAD+ exerts its anti-cancer effects through several mechanisms:
- A study found that NAD+ regulates cell cycle arrest and programmed cell death of malignant cells. [100]
- In human ovarian tumor tissues, NAD+ enhanced the anti-tumor activities of chemotherapeutic drugs. [101]
- A 2018 study published in Frontiers in Oncology found that NAD+ prevented the progression of cancer by stimulating DNA repair. [102]
- A 2019 study reported that targeting NAD+ metabolism can enhance radiation therapy responses in cancer patients. [103]
- A 2015 study published in the Journal of Molecular & Cellular Oncology found that boosting NAD+ can prevent and treat liver cancer. [104]
H. Improves Cardiovascular Health
NAD+ levels are essential for normal heart function and are associated with improved cardiac recovery from injury. Interestingly, SIRT3, one of the signaling proteins of NAD+, can help improve heart health by preventing enlargement of the heart and scarring. [105-107]
There’s increasing evidence supporting the cardiovascular benefits of NAD+:
- In animal models of heart failure, NAD+ improved a multitude of processes needed for cardiovascular function such as the production of energy for cardiomyocytes (heart muscle cells) and reversing vascular dysfunction and oxidative stress. [108-111]
- A cell study reported that NAD+ protected rat heart tissues against apoptosis (programmed cell death). [112]
- In rats with impaired heart function, NAD+ supplementation improved markers of cardiovascular health. [113-116]
- Studies in mice found that NAD+ can stimulate the regeneration of heart muscle cells, reduce left ventricular contractile dysfunction, and prevent heart enlargement. [117-118]
- Rat studies found that higher levels of NAD+ were associated with improved cardiac function. [2, 119]
- Animal studies also found that lower NAD+ levels were associated with mitochondrial dysfunction in heart muscle cells. [120-121]
- A 2015 study published in Nature Reviews found that NAD+ can prevent the progression of obesity through its antioxidant and anti-inflammatory properties. [122]
- In patients with heart failure, 5 to 9 days of oral nicotinamide riboside (NR) supplementation increased NAD+ levels, improved respiratory capacity, and decreased proinflammatory cytokines. [123]
- Studies found that increasing NAD+ levels can protect against heart injury caused by insufficient blood supply and pressure overload and can help reduce the size of dead heart tissue. [124-127]
I. Lowers Blood Pressure
NAD+ activates SIRT1 which in turn increases the production of nitric oxide, a substance that helps widen blood vessels to allow an increase in blood flow. This process reduces the pressure within the blood vessels.
A number of studies support the antihypertensive effects of NAD+:
- In healthy middle-aged and older adults, NAD+ supplementation for 6 weeks reduced blood pressure and arterial stiffness. [128]
- A study found that administration of NAD+-boosting molecules decreased blood pressure in Korean subjects. [129]
- In obese men and women, administration of NAD+ at 1-2 g per day for 6-12 weeks significantly reduced blood pressure. [130-131]
J. Improves Blood Sugar Levels
NAD+ can help improve blood sugar levels by reducing glucose (blood sugar) uptake. This in turn prevents sudden spikes in blood sugar which can be detrimental to health. In addition, NAD+ can also help increase the body’s response to insulin, a hormone that regulates blood sugar.
There’s a good deal of evidence supporting the beneficial effects of NAD+ on blood sugar levels:
- In prediabetic women, NAD+ increased muscle insulin sensitivity. [132]
- In obese mice, increased NAD+ levels improved glucose and lipid homeostasis by increasing the activity of SIRT1 and SIRT3. [133-134]
K. Boosts Immune Function
The age-related shortening of telomeres adversely affects the function of the immune system. These adverse changes can significantly increase the risk for severe infection and even death. Studies suggest that patients with significantly shortened telomeres are at higher risk for different medical conditions such as rheumatoid arthritis and diabetes mellitus (type 1 and type 2). [135-137] Since NAD+ activates SIRT1, it can help achieve longer and more stable telomeres. This has a positive impact on the overall function of the immune system.
Another mechanism that can help improve the immune function is by suppressing or decreasing the inflammatory response. NAD+ has been shown to possess potent anti-inflammatory properties that can help treat or ward off a broad range of inflammatory conditions.
There is a growing body of evidence that NAD+ can help strengthen immune function:
- Treatment of 24-month-old mice with the NAD+ precursor nicotinamide mononucleotide for 1 week significantly reduced the levels of inflammatory markers such as TNFα and IL-6 in the skeletal muscle. [49]
- Oral nicotinamide has been found to be effective in the management of inflammatory lesions associated with acne vulgaris and acne rosacea. [138]
- In mice with brain inflammation, the administration of a NAD+ precursor attenuated the loss of brain cells and improved behavioral deficits. [139]
- In men exposed to ultraviolet radiation, topical nicotinamide application prevented immunosuppression. [140-142]
- Restoring normal NAD+ levels has been found to decrease the severity of immune reaction in patients with COVID-19 infection. [143]
- Studies suggest that NAD+ has significant immunomodulatory effects such as modulation of cytokine action, regulation of the intercellular adhesion molecules, blockage of mast cell degranulation, and inhibition of protease release from leukocytes. [144-146]
L. Improves Liver Health
By activating SIRT1, NAD+ can produce protective effects on the liver. SIRT1 is known to improve liver health by maintaining mitochondrial integrity, improving cholesterol transport, and improving fatty acid homeostasis. [147-149]
Several lines of evidence suggest that NAD+ can help prevent the development of liver diseases:
- A 2016 study found that NAD+ deficiency in the liver increases the risk of non-alcoholic fatty liver disease. [150-151]
- In mice, NAD+ attenuated alcohol-induced liver injury. [152]
- In a mouse model of liver fibrosis, NAD+ prevented liver scarring. [153]
- A 2019 study found that NAD+ protected against aging-induced non-alcoholic fatty liver disease-like liver dysfunction in mice. [154]
- A 2015 study published in Nature Reviews found that NAD+ can prevent the progression of non-alcoholic fatty liver disease by influencing the oxidative stress response, programmed cell death, and inflammatory response. [155]
M. Improves Kidney Health
Reduced levels of NAD+ also reduce sirtuin activity. This process is largely responsible for the age-related decline in kidney function.
Latest studies indicate that NAD+ is beneficial for kidney health:
- A 2019 study published in National Reviews in Nephrology found that NAD+ deficiency could lead to chronic kidney disease. [156]
- A 2017 study also found that NAD+ supplementation can help improve kidney function. [157]
- A 2017 study also found that lower NAD+ levels were associated with a higher incidence of acute kidney injury. [158]
- Studies in mice showed that NAD+ protected against kidney injury caused by chemotherapeutic drugs such as cisplatin and other medications that are toxic to the kidneys. [159-165]
Nicotinamide Adenine Dinucleotide Side Effects
NAD+ side effects are very uncommon. There have been some side effects associated with the use of this drug wherein the patient had one of the issues listed below at some point while being on NAD+. However, these side effects weren’t confirmed to be associated with the treatment and could have been a coincidence and not related to the use of NAD+. Despite this, it was listed as a side effect associated with NAD+ even though these associated side effects are very uncommon.
Side effects associated with NAD+ may include the following:
- Decreased blood levels of phosphorus
- Decreased insulin sensitivity
- Decreased platelets
- Dizziness
- Frontal dull headaches
- Nausea
Reference
-
Monaghan, P., & Haussmann, M. F. (2006). Do telomere dynamics link lifestyle and lifespan?. Trends in ecology & evolution, 21(1), 47–53. https://doi.org/10.1016/j.tree.2005.11.007.
-
Monaghan P. (2010). Telomeres and life histories: the long and the short of it. Annals of the New York Academy of Sciences, 1206, 130–142. https://doi.org/10.1111/j.1749-6632.2010.05705.x.
-
Palacios, J. A., Herranz, D., De Bonis, M. L., Velasco, S., Serrano, M., & Blasco, M. A. (2010). SIRT1 contributes to telomere maintenance and augments global homologous recombination. The Journal of cell biology, 191(7), 1299–1313. https://doi.org/10.1083/jcb.201005160.
-
Wang, Y., Oxer, D., & Hekimi, S. (2015). Mitochondrial function and lifespan of mice with controlled ubiquinone biosynthesis. Nature communications, 6, 6393. https://doi.org/10.1038/ncomms7393.
-
Lanza, I. R., & Nair, K. S. (2010). Mitochondrial function as a determinant of life span. Pflugers Archiv: European journal of physiology, 459(2), 277–289. https://doi.org/10.1007/s00424-009-0724-5.
-
Imai S, Guarente L. NAD+ and sirtuins in aging and disease. Trends Cell Biol. 2014;24(8):464-471. doi:10.1016/j.tcb.2014.04.002.
-
Tang B. L. (2016). Sirt1 and the Mitochondria. Molecules and cells, 39(2), 87–95. https://doi.org/10.14348/molcells.2016.2318.
-
Belenky, P., Racette, F. G., Bogan, K. L., McClure, J. M., Smith, J. S., & Brenner, C. (2007). Nicotinamide riboside promotes Sir2 silencing and extends lifespan via Nrk and Urh1/Pnp1/Meu1 pathways to NAD+. Cell, 129(3), 473–484. https://doi.org/10.1016/j.cell.2007.03.024.
-
Fang, E. F., Kassahun, H., Croteau, D. L., Scheibye-Knudsen, M., Marosi, K., Lu, H., Shamanna, R. A., Kalyanasundaram, S., Bollineni, R. C., Wilson, M. A., Iser, W. B., Wollman, B. N., Morevati, M., Li, J., Kerr, J. S., Lu, Q., Waltz, T. B., Tian, J., Sinclair, D. A., Mattson, M. P., … Bohr, V. A. (2016). NAD+ Replenishment Improves Lifespan and Healthspan in Ataxia Telangiectasia Models via Mitophagy and DNA Repair. Cell metabolism, 24(4), 566–581. https://doi.org/10.1016/j.cmet.2016.09.004.
-
Harlan, B. A., Killoy, K. M., Pehar, M., Liu, L., Auwerx, J., & Vargas, M. R. (2020). Evaluation of the NAD+ biosynthetic pathway in ALS patients and effect of modulating NAD+ levels in hSOD1-linked ALS mouse models. Experimental neurology, 327, 113219. https://doi.org/10.1016/j.expneurol.2020.113219.
-
Zhang, H., Ryu, D., Wu, Y., Gariani, K., Wang, X., Luan, P., D’Amico, D., Ropelle, E. R., Lutolf, M. P., Aebersold, R., Schoonjans, K., Menzies, K. J., & Auwerx, J. (2016). NAD⁺ repletion improves mitochondrial and stem cell function and enhances life span in mice. Science (New York, N.Y.), 352(6292), 1436–1443. https://doi.org/10.1126/science.aaf2693.
-
Peclat, T. R., Thompson, K. L., Warner, G. M., Chini, C., Tarragó, M. G., Mazdeh, D. Z., Zhang, C., Zavala-Solorio, J., Kolumam, G., Liang Wong, Y., Cohen, R. L., & Chini, E. N. (2022). CD38 inhibitor 78c increases mice lifespan and healthspan in a model of chronological aging. Aging cell, 21(4), e13589. https://doi.org/10.1111/acel.13589.
-
Hashimoto, T., Horikawa, M., Nomura, T., & Sakamoto, K. (2010). Nicotinamide adenine dinucleotide extends the lifespan of Caenorhabditis elegans mediated by sir-2.1 and daf-16. Biogerontology, 11(1), 31–43. https://doi.org/10.1007/s10522-009-9225-3.
-
Mouchiroud, L., Houtkooper, R. H., Moullan, N., Katsyuba, E., Ryu, D., Cantó, C., Mottis, A., Jo, Y. S., Viswanathan, M., Schoonjans, K., Guarente, L., & Auwerx, J. (2013). The NAD(+)/Sirtuin Pathway Modulates Longevity through Activation of Mitochondrial UPR and FOXO Signaling. Cell, 154(2), 430–441. https://doi.org/10.1016/j.cell.2013.06.016.
-
Odoh, C. K., Guo, X., Arnone, J. T., Wang, X., & Zhao, Z. K. (2022). The role of NAD and NAD precursors on longevity and lifespan modulation in the budding yeast, Saccharomyces cerevisiae. Biogerontology, 23(2), 169–199. https://doi.org/10.1007/s10522-022-09958-x.
-
Sun, N., Youle, R. J., & Finkel, T. (2016). The Mitochondrial Basis of Aging. Molecular cell, 61(5), 654–666. https://doi.org/10.1016/j.molcel.2016.01.028.
-
Gomes, A. P., Price, N. L., Ling, A. J., Moslehi, J. J., Montgomery, M. K., Rajman, L., White, J. P., Teodoro, J. S., Wrann, C. D., Hubbard, B. P., Mercken, E. M., Palmeira, C. M., de Cabo, R., Rolo, A. P., Turner, N., Bell, E. L., & Sinclair, D. A. (2013). Declining NAD(+) induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell, 155(7), 1624–1638. https://doi.org/10.1016/j.cell.2013.11.037.
-
Das, A., Huang, G. X., Bonkowski, M. S., Longchamp, A., Li, C., Schultz, M. B., Kim, L. J., Osborne, B., Joshi, S., Lu, Y., Treviño-Villarreal, J. H., Kang, M. J., Hung, T. T., Lee, B., Williams, E. O., Igarashi, M., Mitchell, J. R., Wu, L. E., Turner, N., Arany, Z., … Sinclair, D. A. (2018). Impairment of an Endothelial NAD+-H2S Signaling Network Is a Reversible Cause of Vascular Aging. Cell, 173(1), 74–89.e20. https://doi.org/10.1016/j.cell.2018.02.008.
-
Ryu, D., Zhang, H., Ropelle, E. R., Sorrentino, V., Mázala, D. A., Mouchiroud, L., Marshall, P. L., Campbell, M. D., Ali, A. S., Knowels, G. M., Bellemin, S., Iyer, S. R., Wang, X., Gariani, K., Sauve, A. A., Cantó, C., Conley, K. E., Walter, L., Lovering, R. M., Chin, E. R., … Auwerx, J. (2016). NAD+ repletion improves muscle function in muscular dystrophy and counters global PARylation. Science translational medicine, 8(361), 361ra139. https://doi.org/10.1126/scitranslmed.aaf5504.
-
Mills, K. F., Yoshida, S., Stein, L. R., Grozio, A., Kubota, S., Sasaki, Y., Redpath, P., Migaud, M. E., Apte, R. S., Uchida, K., Yoshino, J., & Imai, S. I. (2016). Long-Term Administration of Nicotinamide Mononucleotide Mitigates Age-Associated Physiological Decline in Mice. Cell metabolism, 24(6), 795–806. https://doi.org/10.1016/j.cmet.2016.09.013.
-
Lin, J. B., Kubota, S., Ban, N., Yoshida, M., Santeford, A., Sene, A., Nakamura, R., Zapata, N., Kubota, M., Tsubota, K., Yoshino, J., Imai, S. I., & Apte, R. S. (2016). NAMPT-Mediated NAD(+) Biosynthesis Is Essential for Vision In Mice. Cell reports, 17(1), 69–85. https://doi.org/10.1016/j.celrep.2016.08.073.
-
Khan, N. A., Auranen, M., Paetau, I., Pirinen, E., Euro, L., Forsström, S., Pasila, L., Velagapudi, V., Carroll, C. J., Auwerx, J., & Suomalainen, A. (2014). Effective treatment of mitochondrial myopathy by nicotinamide riboside, a vitamin B3. EMBO molecular medicine, 6(6), 721–731. https://doi.org/10.1002/emmm.201403943.
-
Brown, K. D., Maqsood, S., Huang, J. Y., Pan, Y., Harkcom, W., Li, W., Sauve, A., Verdin, E., & Jaffrey, S. R. (2014). Activation of SIRT3 by the NAD⁺ precursor nicotinamide riboside protects from noise-induced hearing loss. Cell metabolism, 20(6), 1059–1068. https://doi.org/10.1016/j.cmet.2014.11.003.
-
Yoshino, J., Mills, K. F., Yoon, M. J., & Imai, S. (2011). Nicotinamide mononucleotide, a key NAD(+) intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell metabolism, 14(4), 528–536. https://doi.org/10.1016/j.cmet.2011.08.014.
-
Yang, Q., Cong, L., Wang, Y., Luo, X., Li, H., Wang, H., Zhu, J., Dai, S., Jin, H., Yao, G., Shi, S., Hsueh, A. J., & Sun, Y. (2020). Increasing ovarian NAD+ levels improve mitochondrial functions and reverse ovarian aging. Free radical biology & medicine, 156, 1–10. https://doi.org/10.1016/j.freeradbiomed.2020.05.003.
-
Roh, E., Myoung Kang, G., Young Gil, S., Hee Lee, C., Kim, S., Hong, D., Hoon Son, G., & Kim, M. S. (2018). Effects of Chronic NAD Supplementation on Energy Metabolism and Diurnal Rhythm in Obese Mice. Obesity (Silver Spring, Md.), 26(9), 1448–1456. https://doi.org/10.1002/oby.22263.
-
Alegre, J., Rosés, J. M., Javierre, C., Ruiz-Baqués, A., Segundo, M. J., & de Sevilla, T. F. (2010). Nicotinamida adenina dinucleótido (NADH) en pacientes con síndrome de fatiga crónica [Nicotinamide adenine dinucleotide (NADH) in patients with chronic fatigue syndrome]. Revista clinica espanola, 210(6), 284–288. https://doi.org/10.1016/j.rce.2009.09.015.
-
Dehhaghi, M., Panahi, H., Kavyani, B., Heng, B., Tan, V., Braidy, N., & Guillemin, G. J. (2022). The Role of Kynurenine Pathway and NAD+ Metabolism in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Aging and disease, 13(3), 698–711. https://doi.org/10.14336/AD.2021.0824.
-
Castro-Marrero, J., Segundo, M. J., Lacasa, M., Martinez-Martinez, A., Sentañes, R. S., & Alegre-Martin, J. (2021). Effect of Dietary Coenzyme Q10 Plus NADH Supplementation on Fatigue Perception and Health-Related Quality of Life in Individuals with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Prospective, Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients, 13(8), 2658. https://doi.org/10.3390/nu13082658.
-
Castro-Marrero, J., Sáez-Francàs, N., Segundo, M. J., Calvo, N., Faro, M., Aliste, L., Fernández de Sevilla, T., & Alegre, J. (2016). Effect of coenzyme Q10 plus nicotinamide adenine dinucleotide supplementation on maximum heart rate after exercise testing in chronic fatigue syndrome – A randomized, controlled, double-blind trial. Clinical nutrition (Edinburgh, Scotland), 35(4), 826–834. https://doi.org/10.1016/j.clnu.2015.07.010.
-
Castro-Marrero, J., Cordero, M. D., Segundo, M. J., Sáez-Francàs, N., Calvo, N., Román-Malo, L., Aliste, L., Fernández de Sevilla, T., & Alegre, J. (2015). Does oral coenzyme Q10 plus NADH supplementation improve fatigue and biochemical parameters in chronic fatigue syndrome?. Antioxidants & redox signaling, 22(8), 679–685. https://doi.org/10.1089/ars.2014.6181.
-
Mach, J., Midgley, A. W., Dank, S., Grant, R. S., & Bentley, D. J. (2010). The effect of antioxidant supplementation on fatigue during exercise: potential role for NAD+(H). Nutrients, 2(3), 319–329. https://doi.org/10.3390/nu2030319.
-
Santaella, M. L., Font, I., & Disdier, O. M. (2004). Comparison of oral nicotinamide adenine dinucleotide (NADH) versus conventional therapy for chronic fatigue syndrome. Puerto Rico health sciences journal, 23(2), 89–93.
-
Stein LR, Imai S. The dynamic regulation of NAD metabolism in mitochondria. Trends EndocrinolMetab. 2012;23(9):420-428. doi:10.1016/j.tem.2012.06.005.
-
Garten A, Schuster S, Penke M, Gorski T, de Giorgis T, Kiess W. Physiological and pathophysiological roles of NAMPT and NAD metabolism. Nat Rev Endocrinol. 2015;11(9):535-546. doi:10.1038/nrendo.2015.117.
-
Uddin GM, Youngson NA, Sinclair DA, Morris MJ. Head to Head Comparison of Short-Term Treatment with the NAD(+) Precursor Nicotinamide Mononucleotide (NMN) and 6 Weeks of Exercise in Obese Female Mice. Front Pharmacol. 2016;7:258. Published 2016 Aug 19. doi:10.3389/fphar.2016.00258.
-
Rappou E, Jukarainen S, Rinnankoski-Tuikka R et al (2016) Weight loss is associated with increased NAD+/SIRT1 expression but reduced PARP activity in white adipose tissue. J ClinEndocrinolMetab 101(3):1263–1273. https://doi.org/10.1210/jc.2015-3054.
-
Cantó C, Houtkooper RH, Pirinen E, et al. The NAD(+) precursor nicotinamideriboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab. 2012;15(6):838-847. doi:10.1016/j.cmet.2012.04.022.
-
Crisol BM, Veiga CB, Lenhare L, et al. Nicotinamideriboside induces a thermogenic response in lean mice. Life Sci. 2018;211:1-7. doi:10.1016/j.lfs.2018.09.015.
-
Jukarainen S, Heinonen S, Rämö JT, et al. Obesity Is Associated With Low NAD(+)/SIRT Pathway Expression in Adipose Tissue of BMI-Discordant Monozygotic Twins. J ClinEndocrinolMetab. 2016;101(1):275-283. doi:10.1210/jc.2015-3095.
-
Yamaguchi S, Yoshino J. Adipose tissue NAD+ biology in obesity and insulin resistance: From mechanism to therapy. Bioessays. 2017;39(5):10.1002/bies.201600227. doi:10.1002/bies.201600227.
-
Yoshino J, Mills KF, Yoon MJ, Imai S. Nicotinamide mononucleotide, a key NAD (+) intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab. 2011;14(4):528–536. doi: 10.1016/j.cmet.2011.08.014.
-
Trammell SA, Weidemann BJ, Chadda A, Yorek MS, Holmes A, Coppey LJ, Obrosov A, Kardon RH, Yorek MA, Brenner C. Nicotinamideriboside opposes type 2 diabetes and neuropathy in mice. Sci Rep. 2016;6:26933. doi: 10.1038/srep26933.
-
Frederick DW, Davis JG, Davila A, Jr, Agarwal B, Michan S, Puchowicz MA, Nakamaru-Ogiso E, Baur JA. Increasing NAD synthesis in muscle via nicotinamidephosphoribosyltransferase is not sufficient to promote oxidative metabolism. J Biol Chem. 2015;290(3):1546–1558. doi: 10.1074/jbc.M114.579565.
-
Sasaki T, Kikuchi O, Shimpuku M, Susanti VY, Yokota-Hashimoto H, Taguchi R, Shibusawa N, Sato T, et al. Hypothalamic SIRT1 prevents age-associated weight gain by improving leptin sensitivity in mice. Diabetologia. 2014;57(4):819–831. doi: 10.1007/s00125-013-3140-5.
-
Yamaguchi S, Franczyk MP, Chondronikola M, et al. Adipose tissue NAD+ biosynthesis is required for regulating adaptive thermogenesis and whole-body energy homeostasis in mice. ProcNatlAcadSci U S A. 2019;116(47):23822-23828. doi:10.1073/pnas.1909917116.
-
Mills, K. F., Yoshida, S., Stein, L. R., Grozio, A., Kubota, S., Sasaki, Y., et al. (2016). Long-term administration of nicotinamide mononucleotide mitigates age-associated physiological decline in mice. Cell Metab. 24, 795–806. doi: 10.1016/j.cmet.2016.09.013.
-
Cantó, C., Houtkooper, R. H., Pirinen, E., Youn, D. Y., Oosterveer, M. H., Cen, Y., Fernandez-Marcos, P. J., Yamamoto, H., Andreux, P. A., Cettour-Rose, P., Gademann, K., Rinsch, C., Schoonjans, K., Sauve, A. A., & Auwerx, J. (2012). The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell metabolism, 15(6), 838–847. https://doi.org/10.1016/j.cmet.2012.04.022.
-
Gomes, A. P., Price, N. L., Ling, A. J., Moslehi, J. J., Montgomery, M. K., Rajman, L., White, J. P., Teodoro, J. S., Wrann, C. D., Hubbard, B. P., Mercken, E. M., Palmeira, C. M., de Cabo, R., Rolo, A. P., Turner, N., Bell, E. L., & Sinclair, D. A. (2013). Declining NAD(+) induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell, 155(7), 1624–1638. https://doi.org/10.1016/j.cell.2013.11.037.
-
Yaku K, Okabe K, Hikosaka K, Nakagawa T. NAD Metabolism in Cancer Therapeutics. Front Oncol. 2018;8:622. Published 2018 Dec 12. doi:10.3389/fonc.2018.00622.
-
Lewis JE, Singh N, Holmila RJ, et al. Targeting NAD+ Metabolism to Enhance Radiation Therapy Responses. SeminRadiatOncol. 2019;29(1):6-15. doi:10.1016/j.semradonc.2018.10.009.
-
Djouder N. Boosting NAD(+) for the prevention and treatment of liver cancer. Mol Cell Oncol. 2015;2(4):e1001199. Published 2015 Feb 3. doi:10.1080/23723556.2014.1001199.
-
Elhassan YS, Kluckova K, Fletcher RS, et al. NicotinamideRiboside Augments the Aged Human Skeletal Muscle NAD+ Metabolome and Induces Transcriptomic and Anti-inflammatory Signatures. Cell Rep. 2019;28(7):1717-1728.e6. doi:10.1016/j.celrep.2019.07.043.
-
Mendelsohn AR, Larrick JW. Partial reversal of skeletal muscle aging by restoration of normal NAD⁺ levels. Rejuvenation Res. 2014;17(1):62-69. doi:10.1089/rej.2014.1546.
-
Goody MF, Henry CA. A need for NAD+ in muscle development, homeostasis, and aging. Skelet Muscle. 2018;8(1):9. Published 2018 Mar 7. doi:10.1186/s13395-018-0154-1.
-
Lautrup, S., Sinclair, D. A., Mattson, M. P., & Fang, E. F. (2019). NAD+ in Brain Aging and Neurodegenerative Disorders. Cell metabolism, 30(4), 630–655. https://doi.org/10.1016/j.cmet.2019.09.001.
-
Mao, K., & Zhang, G. (2022). The role of PARP1 in neurodegenerative diseases and aging. The FEBS journal, 289(8), 2013–2024. https://doi.org/10.1111/febs.15716.
-
Ying W. (2007). NAD+ and NADH in brain functions, brain diseases and brain aging. Frontiers in bioscience: a journal and virtual library, 12, 1863–1888. https://doi.org/10.2741/2194.
-
Lloret, A., & Beal, M. F. (2019). PGC-1α, Sirtuins and PARPs in Huntington’s Disease and Other Neurodegenerative Conditions: NAD+ to Rule Them All. Neurochemical research, 44(10), 2423–2434. https://doi.org/10.1007/s11064-019-02809-1.
-
Yang H, Yang T, Baur JA, et al. Nutrient-sensitive mitochondrial NAD+ levels dictate cell survival. Cell. 2007;130(6):1095-1107. doi:10.1016/j.cell.2007.07.035.
-
Verdin E. NAD⁺ in aging, metabolism, and neurodegeneration. Science. 2015;350(6265):1208-1213. doi:10.1126/science.aac4854.
-
Ying W. NAD+ and NADH in brain functions, brain diseases and brain aging. Front Biosci. 2007;12:1863-1888. Published 2007 Jan 1. doi:10.2741/2194.
-
Belenky P, Racette FG, Bogan KL, McClure JM, Smith JS, Brenner C. Nicotinamideriboside promotes Sir2 silencing and extends lifespan via Nrk and Urh1/Pnp1/Meu1 pathways to NAD+. Cell. 2007 May 4;129(3):473-84.
-
Rajman L, Chwalek K, Sinclair DA. Therapeutic Potential of NAD-Boosting Molecules: The In Vivo Evidence. Cell Metab. 2018;27(3):529-547. doi:10.1016/j.cmet.2018.02.011.
-
Massudi H, Grant R, Braidy N, Guest J, Farnsworth B, Guillemin GJ. Age-associated changes in oxidative stress and NAD+ metabolism in human tissue. PLoS One. 2012;7(7):e42357. doi:10.1371/journal.pone.0042357.
-
Braidy N, Liu Y. NAD+ therapy in age-related degenerative disorders: A benefit/risk analysis. ExpGerontol. 2020;132:110831. doi:10.1016/j.exger.2020.110831.
-
Schultz MB, Sinclair DA. Why NAD(+) Declines during Aging: It’s Destroyed. Cell Metab. 2016;23(6):965-966. doi:10.1016/j.cmet.2016.05.022.
-
Yaku K, Okabe K, Nakagawa T. NAD metabolism: Implications in aging and longevity. Ageing Res Rev. 2018;47:1-17. doi:10.1016/j.arr.2018.05.006.
-
Cantó C, Menzies KJ, Auwerx J. NAD(+) Metabolism and the Control of Energy Homeostasis: A Balancing Act between Mitochondria and the Nucleus. Cell Metab. 2015;22(1):31-53. doi:10.1016/j.cmet.2015.05.023.
-
Zhang N, Sauve AA. Regulatory Effects of NAD+ Metabolic Pathways on Sirtuin Activity. ProgMolBiolTransl Sci. 2018;154:71-104. doi:10.1016/bs.pmbts.2017.11.012.
-
Connell, N.J., Houtkooper, R.H. &Schrauwen, P. NAD+ metabolism as a target for metabolic health: have we found the silver bullet?.Diabetologia 62, 888–899 (2019). https://doi.org/10.1007/s00125-019-4831-3.
-
Elhassan YS, Philp AA, Lavery GG. Targeting NAD+ in Metabolic Disease: New Insights Into an Old Molecule. J Endocr Soc. 2017;1(7):816-835. Published 2017 May 15. doi:10.1210/js.2017-00092.
-
Okabe K, Yaku K, Tobe K, Nakagawa T. Implications of altered NAD metabolism in metabolic disorders. J Biomed Sci. 2019;26(1):34. Published 2019 May 11. doi:10.1186/s12929-019-0527-8.
-
Prolla TA, Denu JM. NAD+ deficiency in age-related mitochondrial dysfunction. Cell Metab. 2014;19(2):178-180. doi:10.1016/j.cmet.2014.01.005.
-
Seo KS, Kim JH, Min KN, et al. KL1333, a Novel NAD+ Modulator, Improves Energy Metabolism and Mitochondrial Dysfunction in MELAS Fibroblasts. Front Neurol. 2018;9:552. Published 2018 Jul 5. doi:10.3389/fneur.2018.00552.
-
Goody MF, Henry CA. A need for NAD+ in muscle development, homeostasis, and aging. Skelet Muscle. 2018;8(1):9. Published 2018 Mar 7. doi:10.1186/s13395-018-0154-1.
-
Lightowlers RN, Chrzanowska-Lightowlers ZM. Salvaging hope: Is increasing NAD(+) a key to treating mitochondrial myopathy?. EMBO Mol Med. 2014;6(6):705-707. doi:10.15252/emmm.201404179.
-
Srivastava S. Emerging therapeutic roles for NAD(+) metabolism in mitochondrial and age-related disorders. ClinTransl Med. 2016;5(1):25. doi:10.1186/s40169-016-0104-7.
-
Yang Y, Sauve AA. NAD(+) metabolism: Bioenergetics, signaling and manipulation for therapy. BiochimBiophysActa. 2016;1864(12):1787-1800. doi:10.1016/j.bbapap.2016.06.014.
-
Liu D, Pitta M, Mattson MP. Preventing NAD(+) depletion protects neurons against excitotoxicity: bioenergetic effects of mild mitochondrial uncoupling and caloric restriction. Ann N Y Acad Sci. 2008;1147:275-282. doi:10.1196/annals.1427.028.
-
Brennan AM, Connor JA, Shuttleworth CW. NAD(P)H fluorescence transients after synaptic activity in brain slices: predominant role of mitochondrial function. J. Cereb. Blood Flow Metab. 2006;26:1389–1406.
-
Yaku, K., Okabe, K., Gulshan, M. et al. Metabolism and biochemical properties of nicotinamide adenine dinucleotide (NAD) analogs, nicotinamide guanine dinucleotide (NGD) and nicotinamide hypoxanthine dinucleotide (NHD). Sci Rep 9, 13102 (2019). https://doi.org/10.1038/s41598-019-49547-6.
-
Fricker RA, Green EL, Jenkins SI, Griffin SM. The Influence of Nicotinamide on Health and Disease in the Central Nervous System. Int J Tryptophan Res. 2018;11:1178646918776658. Published 2018 May 21. doi:10.1177/1178646918776658.
-
Grant R, Berg J, Mestayer R, et al. A Pilot Study Investigating Changes in the Human Plasma and Urine NAD+ MetabolomeDuring a 6 Hour Intravenous Infusion of NAD. Front Aging Neurosci. 2019;11:257. Published 2019 Sep 12. doi:10.3389/fnagi.2019.00257.
-
Lloret A, Beal MF. PGC-1α, Sirtuins and PARPs in Huntington’s Disease and Other Neurodegenerative Conditions: NAD+ to Rule Them All. Neurochem Res. 2019;44(10):2423-2434. doi:10.1007/s11064-019-02809-1.
-
Ying W. NAD+ and NADH in brain functions, brain diseases and brain aging. Front Biosci. 2007;12:1863-1888. Published 2007 Jan 1. doi:10.2741/2194.
-
Liu D, Gharavi R, Pitta M, Gleichmann M, Mattson MP. Nicotinamide prevents NAD+ depletion and protects neurons against excitotoxicity and cerebral ischemia: NAD+ consumption by SIRT1 may endanger energetically compromised neurons. Neuromolecular Med. 2009;11(1):28-42. doi:10.1007/s12017-009-8058-1.
-
Liu J, Yang B, Zhou P, et al. Nicotinamide adenine dinucleotide suppresses epileptogenesis at an early stage. Sci Rep. 2017;7(1):7321. Published 2017 Aug 4. doi:10.1038/s41598-017-07343-0.
-
Alano CC, Garnier P, Ying W, Higashi Y, Kauppinen TM, Swanson RA. NAD+ depletion is necessary and sufficient for poly(ADP-ribose) polymerase-1-mediated neuronal death. J Neurosci. 2010;30(8):2967-2978. doi:10.1523/JNEUROSCI.5552-09.2010.
-
Hou Y, Lautrup S, Cordonnier S, et al. NAD+ supplementation normalizes key Alzheimer’s features and DNA damage responses in a new AD mouse model with introduced DNA repair deficiency. ProcNatlAcadSci U S A. 2018;115(8):E1876-E1885. doi:10.1073/pnas.1718819115.
-
Xing S, Hu Y, Huang X, Shen D, Chen C. Nicotinamidephosphoribosyltransferase‑related signaling pathway in early Alzheimer’s disease mouse models. Mol Med Rep. 2019;20(6):5163-5171. doi:10.3892/mmr.2019.10782.
-
Braidy N, Grant R, Sachdev PS. Nicotinamide adenine dinucleotide and its related precursors for the treatment of Alzheimer’s disease. CurrOpin Psychiatry. 2018;31(2):160-166. doi:10.1097/YCO.0000000000000394.
-
Demarin V, Podobnik SS, Storga-Tomic D, Kay G. Treatment of Alzheimer’s disease with stabilized oral nicotinamide adenine dinucleotide: a randomized, double-blind study. Drugs ExpClin Res. 2004;30(1):27-33.
-
Fang EF, Hou Y, Lautrup S, et al. NAD+ augmentation restores mitophagy and limits accelerated aging in Werner syndrome. Nat Commun. 2019;10(1):5284. Published 2019 Nov 21. doi:10.1038/s41467-019-13172-8.
-
Dong, Y., Sameni, S., Digman, M.A. et al. Reversibility of Age-related Oxidized Free NADH Redox States in Alzheimer’s Disease Neurons by Imposed External Cys/CySS Redox Shifts. Sci Rep 9, 11274 (2019). https://doi.org/10.1038/s41598-019-47582-x.
-
Sorrentino, V., Romani, M., Mouchiroud, L., Beck, J. S., Zhang, H., D’Amico, D., Moullan, N., Potenza, F., Schmid, A. W., Rietsch, S., Counts, S. E., & Auwerx, J. (2017). Enhancing mitochondrial proteostasis reduces amyloid-β proteotoxicity. Nature, 552(7684), 187–193. https://doi.org/10.1038/nature25143.
-
Wu, L. E., Gomes, A. P., & Sinclair, D. A. (2014). Geroncogenesis: metabolic changes during aging as a driver of tumorigenesis. Cancer cell, 25(1), 12–19. https://doi.org/10.1016/j.ccr.2013.12.005.
-
Firestein, R., Blander, G., Michan, S., Oberdoerffer, P., Ogino, S., Campbell, J., Bhimavarapu, A., Luikenhuis, S., de Cabo, R., Fuchs, C., Hahn, W. C., Guarente, L. P., & Sinclair, D. A. (2008). The SIRT1 deacetylase suppresses intestinal tumorigenesis and colon cancer growth. PloS one, 3(4), e2020. https://doi.org/10.1371/journal.pone.0002020.
-
Sebastián, C., Zwaans, B. M., Silberman, D. M., Gymrek, M., Goren, A., Zhong, L., Ram, O., Truelove, J., Guimaraes, A. R., Toiber, D., Cosentino, C., Greenson, J. K., MacDonald, A. I., McGlynn, L., Maxwell, F., Edwards, J., Giacosa, S., Guccione, E., Weissleder, R., Bernstein, B. E., … Mostoslavsky, R. (2012). The histone deacetylase SIRT6 is a tumor suppressor that controls cancer metabolism. Cell, 151(6), 1185–1199. https://doi.org/10.1016/j.cell.2012.10.047.
-
Lee MK, Cheong HS, Koh Y, Ahn KS, Yoon SS, Shin HD. Genetic Association of PARP15 Polymorphisms with Clinical Outcome of Acute Myeloid Leukemia in a Korean Population. Genet Test Mol Biomarkers. 2016;20:696–701.
-
Dollerup O.L., Christensen B., Svart M., Schmidt M.S., Sulek K., Ringgaard S., Stødkilde-Jørgensen H., Møller N., Brenner C., Treebak J.T., Jessen N. A randomized placebo-controlled clinical trial of nicotinamideriboside in obese men: safety, insulin-sensitivity, and lipid-mobilizing effects. Am. J. Clin. Nutr. 2018;108:343–353.
-
Martens C.R., Denman B.A., Mazzo M.R., Armstrong M.L., Reisdorph N., McQueen M.B., Chonchol M., Seals D.R. Chronic nicotinamideriboside supplementation is well-tolerated and elevates NAD+ in healthy middle-aged and older adults. Nat. Commun. 2018;9:1286.
-
Yaku K, Okabe K, Hikosaka K, Nakagawa T. NAD Metabolism in Cancer Therapeutics. Front Oncol. 2018;8:622. Published 2018 Dec 12. doi:10.3389/fonc.2018.00622.
-
Available from https://www.biorxiv.org/content/10.1101/2020.03.21.001123v1.
-
Sundaresan, N. R., Gupta, M., Kim, G., Rajamohan, S. B., Isbatan, A., & Gupta, M. P. (2009). Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. The Journal of clinical investigation, 119(9), 2758–2771. https://doi.org/10.1172/JCI39162.
-
Hafner, A. V., Dai, J., Gomes, A. P., Xiao, C. Y., Palmeira, C. M., Rosenzweig, A., & Sinclair, D. A. (2010). Regulation of the mPTP by SIRT3-mediated deacetylation of CypD at lysine 166 suppresses age-related cardiac hypertrophy. Aging, 2(12), 914–923. https://doi.org/10.18632/aging.100252.
-
Sundaresan, N. R., Gupta, M., Kim, G., Rajamohan, S. B., Isbatan, A., & Gupta, M. P. (2009). Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. The Journal of clinical investigation, 119(9), 2758–2771. https://doi.org/10.1172/JCI39162.
-
Nacarelli, T., Lau, L., Fukumoto, T., Zundell, J., Fatkhutdinov, N., Wu, S., Aird, K. M., Iwasaki, O., Kossenkov, A. V., Schultz, D., Noma, K. I., Baur, J. A., Schug, Z., Tang, H. Y., Speicher, D. W., David, G., & Zhang, R. (2019). NAD+ metabolism governs the proinflammatory senescence-associated secretome. Nature cell biology, 21(3), 397–407. https://doi.org/10.1038/s41556-019-0287-4.
-
Gong B, Pan Y, Vempati P, et al. Nicotinamideriboside restores cognition through an upregulation of proliferator-activated receptor-γ coactivator 1α regulated β-secretase 1 degradation and mitochondrial gene expression in Alzheimer’s mouse models. Neurobiol Aging. 2013;34(6):1581-1588. doi:10.1016/j.neurobiolaging.2012.12.005.
-
Matasic DS, Brenner C, London B. Emerging potential benefits of modulating NAD+ metabolism in cardiovascular disease. Am J Physiol Heart Circ Physiol. 2018;314(4):H839-H852. doi:10.1152/ajpheart.00409.2017.
-
Alano CC, Tran A, Tao R, Ying W, Karliner JS, Swanson RA. Differences among cell types in NAD+ compartmentalization: a comparison of neurons, astrocytes, and cardiac myocytes. J Neurosci Res 85: 3378–3385, 2007. doi:10.1002/jnr.21479.
-
de Picciotto NE, Gano LB, Johnson LC, Martens CR, Sindler AL, Mills KF, Imai S, Seals DR. Nicotinamide mononucleotide supplementation reverses vascular dysfunction and oxidative stress with aging in mice. Aging Cell 15: 522–530, 2016. doi:10.1111/acel.12461.
-
Alano CC, Tran A, Tao R, Ying W, Karliner JS, Swanson RA. Differences among cell types in NAD+ compartmentalization: a comparison of neurons, astrocytes, and cardiac myocytes. J Neurosci Res 85: 3378–3385, 2007. doi:10.1002/jnr.21479.
-
Liu L, Wang P, Liu X, He D, Liang C, Yu Y. Exogenous NAD(+) supplementation protects H9c2 cardiac myoblasts against hypoxia/reoxygenation injury via Sirt1-p53 pathway. FundamClinPharmacol. 2014;28(2):180-189. doi:10.1111/fcp.12016.
-
Ryu D, Zhang H, Ropelle ER, Sorrentino V, Mazala DA, Mouchiroud L, Marshall PL, Campbell MD, Ali AS, Knowels GM, et al. NAD+ repletion improves muscle function in muscular dystrophy and counters global PARylation. SciTransl Med. 2016;8:361ra139.
-
Xu W, Barrientos T, Mao L, Rockman HA, Sauve AA, Andrews NC. Lethal Cardiomyopathy in Mice Lacking Transferrin Receptor in the Heart. Cell Rep. 2015;13:533–545.
-
Chan PK, Torres R, Yandim C, Law PP, Khadayate S, Mauri M, Grosan C, Chapman-Rothe N, Giunti P, Pook M, et al. Heterochromatinization induced by GAA-repeat hyperexpansion in Friedreich’s ataxia can be reduced upon HDAC inhibition by vitamin B3. Hum Mol Genet. 2013;22:2662–2675.
-
Martin AS, Abraham DM, Hershberger KA, Bhatt DP, Mao L, Cui H, Liu J, Liu X, Muehlbauer MJ, Grimsrud PA, et al. Nicotinamide mononucleotide requires SIRT3 to improve cardiac function and bioenergetics in a Friedreich’s ataxia cardiomyopathy model. JCI Insight. 2017;2.
-
Katsyuba, E., Romani, M., Hofer, D. et al. NAD+ homeostasis in health and disease. Nat Metab 2, 9–31 (2020). https://doi.org/10.1038/s42255-019-0161-5.
-
Walker MA, Tian R. Raising NAD in Heart Failure: Time to Translate?. Circulation. 2018;137(21):2274-2277. doi:10.1161/CIRCULATIONAHA.117.032626.
-
Airhart SE, Shireman LM, Risler LJ, et al. An open-label, non-randomized study of the pharmacokinetics of the nutritional supplement nicotinamideriboside (NR) and its effects on blood NAD+ levels in healthy volunteers. PLoS One. 2017;12(12):e0186459. Published 2017 Dec 6. doi:10.1371/journal.pone.0186459.
-
Lee CF, Caudal A, Abell L, NaganaGowda GA, Tian R. Targeting NAD+ Metabolism as Interventions for Mitochondrial Disease. Sci Rep. 2019;9(1):3073. Published 2019 Feb 28. doi:10.1038/s41598-019-39419-4.
-
Zhou, B., Wang, D. D., Qiu, Y., Airhart, S., Liu, Y., Stempien-Otero, A., O’Brien, K. D., & Tian, R. (2020). Boosting NAD level suppresses inflammatory activation of PBMCs in heart failure. The Journal of clinical investigation, 130(11), 6054–6063. https://doi.org/10.1172/JCI138538.
-
Hsu, C. P., Oka, S., Shao, D., Hariharan, N., & Sadoshima, J. (2009). Nicotinamide phosphoribosyltransferase regulates cell survival through NAD+ synthesis in cardiac myocytes. Circulation research, 105(5), 481–491. https://doi.org/10.1161/CIRCRESAHA.109.203703.
-
Karamanlidis, G., Lee, C. F., Garcia-Menendez, L., Kolwicz, S. C., Jr, Suthammarak, W., Gong, G., Sedensky, M. M., Morgan, P. G., Wang, W., & Tian, R. (2013). Mitochondrial complex I deficiency increases protein acetylation and accelerates heart failure. Cell metabolism, 18(2), 239–250. https://doi.org/10.1016/j.cmet.2013.07.002.
-
Pillai, J. B., Isbatan, A., Imai, S., & Gupta, M. P. (2005). Poly(ADP-ribose) polymerase-1-dependent cardiac myocyte cell death during heart failure is mediated by NAD+ depletion and reduced Sir2alpha deacetylase activity. The Journal of biological chemistry, 280(52), 43121–43130. https://doi.org/10.1074/jbc.M506162200.
-
Yamamoto, T., Byun, J., Zhai, P., Ikeda, Y., Oka, S., & Sadoshima, J. (2014). Nicotinamide mononucleotide, an intermediate of NAD+ synthesis, protects the heart from ischemia and reperfusion. PloS one, 9(6), e98972. https://doi.org/10.1371/journal.pone.0098972.
-
Mattagajasingh, I., Kim, C. S., Naqvi, A., Yamamori, T., Hoffman, T. A., Jung, S. B., DeRicco, J., Kasuno, K., & Irani, K. (2007). SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proceedings of the National Academy of Sciences of the United States of America, 104(37), 14855–14860. https://doi.org/10.1073/pnas.0704329104.
-
Uddin, G. M., Youngson, N. A., Doyle, B. M., Sinclair, D. A., and Morris, M. J. (2017). Nicotinamide mononucleotide (NMN) supplementation ameliorates the impact of maternal obesity in mice: comparison with exercise. Sci. Rep. 7:15063. doi: 10.1038/s41598-017-14866-z.
-
Porter LC, Franczyk MP, Pietka T, et al. NAD+-dependent deacetylase SIRT3 in adipocytes is dispensable for maintaining normal adipose tissue mitochondrial function and whole body metabolism. Am J PhysiolEndocrinolMetab. 2018;315(4):E520-E530. doi:10.1152/ajpendo.00057.2018.
-
Martens CR, Denman BA, Mazzo MR, et al. Chronic nicotinamideriboside supplementation is well-tolerated and elevates NAD+ in healthy middle-aged and older adults. Nat Commun. 2018;9(1):1286. Published 2018 Mar 29. doi:10.1038/s41467-018-03421-7.
-
Yoshino, M., Yoshino, J., Kayser, B. D., Patti, G. J., Franczyk, M. P., Mills, K. F., Sindelar, M., Pietka, T., Patterson, B. W., Imai, S. I., & Klein, S. (2021). Nicotinamide mononucleotide increases muscle insulin sensitivity in prediabetic women. Science (New York, N.Y.), 372(6547), 1224–1229. https://doi.org/10.1126/science.abe9985.
-
Camacho-Pereira, J., Tarragó, M. G., Chini, C., Nin, V., Escande, C., Warner, G. M., Puranik, A. S., Schoon, R. A., Reid, J. M., Galina, A., & Chini, E. N. (2016). CD38 Dictates Age-Related NAD Decline and Mitochondrial Dysfunction through an SIRT3-Dependent Mechanism. Cell metabolism, 23(6), 1127–1139. https://doi.org/10.1016/j.cmet.2016.05.006.
-
Escande, C., Nin, V., Price, N. L., Capellini, V., Gomes, A. P., Barbosa, M. T., O’Neil, L., White, T. A., Sinclair, D. A., & Chini, E. N. (2013). Flavonoid apigenin is an inhibitor of the NAD+ ase CD38: implications for cellular NAD+ metabolism, protein acetylation, and treatment of metabolic syndrome. Diabetes, 62(4), 1084–1093. https://doi.org/10.2337/db12-1139.
-
Koetz, K., Bryl, E., Spickschen, K., O’Fallon, W. M., Goronzy, J. J., & Weyand, C. M. (2000). T cell homeostasis in patients with rheumatoid arthritis. Proceedings of the National Academy of Sciences of the United States of America, 97(16), 9203–9208. https://doi.org/10.1073/pnas.97.16.9203.
-
Fyhrquist, F., Tiitu, A., Saijonmaa, O., Forsblom, C., Groop, P. H., & FinnDiane Study Group (2010). Telomere length and progression of diabetic nephropathy in patients with type 1 diabetes. Journal of internal medicine, 267(3), 278–286. https://doi.org/10.1111/j.1365-2796.2009.02139.x.
-
Testa, R., Olivieri, F., Sirolla, C., Spazzafumo, L., Rippo, M. R., Marra, M., Bonfigli, A. R., Ceriello, A., Antonicelli, R., Franceschi, C., Castellucci, C., Testa, I., & Procopio, A. D. (2011). Leukocyte telomere length is associated with complications of type 2 diabetes mellitus. Diabetic medicine : a journal of the British Diabetic Association, 28(11), 1388–1394. https://doi.org/10.1111/j.1464-5491.2011.03370.x.
-
Niren N. M. (2006). Pharmacologic doses of nicotinamide in the treatment of inflammatory skin conditions: a review. Cutis, 77(1 Suppl), 11–16.
-
Kaneko, S., Wang, J., Kaneko, M., Yiu, G., Hurrell, J. M., Chitnis, T., Khoury, S. J., & He, Z. (2006). Protecting axonal degeneration by increasing nicotinamide adenine dinucleotide levels in experimental autoimmune encephalomyelitis models. The Journal of neuroscience : the official journal of the Society for Neuroscience, 26(38), 9794–9804. https://doi.org/10.1523/JNEUROSCI.2116-06.2006.
-
Damian, D. L., Patterson, C. R., Stapelberg, M., Park, J., Barnetson, R. S., & Halliday, G. M. (2008). UV radiation-induced immunosuppression is greater in men and prevented by topical nicotinamide. The Journal of investigative dermatology, 128(2), 447–454. https://doi.org/10.1038/sj.jid.5701058.
-
Gensler H. L. (1997). Prevention of photoimmunosuppression and photocarcinogenesis by topical nicotinamide. Nutrition and cancer, 29(2), 157–162. https://doi.org/10.1080/01635589709514618.
-
Yiasemides, E., Sivapirabu, G., Halliday, G. M., Park, J., & Damian, D. L. (2009). Oral nicotinamide protects against ultraviolet radiation-induced immunosuppression in humans. Carcinogenesis, 30(1), 101–105. https://doi.org/10.1093/carcin/bgn248.
-
Omran, H. M., & Almaliki, M. S. (2020). Influence of NAD+ as an ageing-related immunomodulator on COVID 19 infection: A hypothesis. Journal of infection and public health, 13(9), 1196–1201. https://doi.org/10.1016/j.jiph.2020.06.004.
-
Hiromatsu, Y., Yang, D., Miyake, I., Koga, M., Kameo, J., Sato, M., Inoue, Y., & Nonaka, K. (1998). Nicotinamide decreases cytokine-induced activation of orbital fibroblasts from patients with thyroid-associated ophthalmopathy. The Journal of clinical endocrinology and metabolism, 83(1), 121–124. https://doi.org/10.1210/jcem.83.1.4478.
-
Hiromatsu, Y., Sato, M., Tanaka, K., Ishisaka, N., Kamachi, J., & Nonaka, K. (1993). Inhibitory effects of nicotinamide on intercellular adhesion molecule-1 expression on cultured human thyroid cells. Immunology, 80(2), 330–332.
-
Silwal P., Shin K., Choi S., Namgung U., Lee C.Y., Heo J.-Y.-Y. Tryptophan negatively regulates IgE-mediated mast cell activation. Korean J Phys Anthropol. 2017;30:53. doi: 10.11637/kjpa.2017.30.2.53.
-
Picard, F., Kurtev, M., Chung, N., Topark-Ngarm, A., Senawong, T., Machado De Oliveira, R., Leid, M., McBurney, M. W., & Guarente, L. (2004). Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature, 429(6993), 771–776. https://doi.org/10.1038/nature02583.
-
Rodgers, J. T., Lerin, C., Haas, W., Gygi, S. P., Spiegelman, B. M., & Puigserver, P. (2005). Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature, 434(7029), 113–118. https://doi.org/10.1038/nature03354.
-
Yang, F., Vought, B. W., Satterlee, J. S., Walker, A. K., Jim Sun, Z. Y., Watts, J. L., DeBeaumont, R., Saito, R. M., Hyberts, S. G., Yang, S., Macol, C., Iyer, L., Tjian, R., van den Heuvel, S., Hart, A. C., Wagner, G., & Näär, A. M. (2006). An ARC/Mediator subunit required for SREBP control of cholesterol and lipid homeostasis. Nature, 442(7103), 700–704. https://doi.org/10.1038/nature04942.
-
Remie CME, Roumans KHM, Moonen MPB, et al. Nicotinamideriboside supplementation alters body composition and skeletal muscle acetylcarnitine concentrations in healthy obese humans [published online ahead of print, 2020 Apr 22]. Am J ClinNutr. 2020;nqaa072. doi:10.1093/ajcn/nqaa072.
-
deGuia RM, Agerholm M, Nielsen TS, et al. Aerobic and resistance exercise training reverses age-dependent decline in NAD+ salvage capacity in human skeletal muscle. Physiol Rep. 2019;7(12):e14139. doi:10.14814/phy2.14139.
-
Ryu D, Zhang H, Ropelle ER, et al. NAD+ repletion improves muscle function in muscular dystrophy and counters global PARylation. SciTransl Med. 2016;8(361):361ra139. doi:10.1126/scitranslmed.aaf5504.
-
Zhou CC, Yang X, Hua X, et al. Hepatic NAD(+) deficiency as a therapeutic target for non-alcoholic fatty liver disease in ageing. Br J Pharmacol. 2016;173(15):2352-2368. doi:10.1111/bph.13513.
-
Guarino M, Dufour JF. Nicotinamide and NAFLD: Is There Nothing New Under the Sun?. Metabolites. 2019;9(9):180. Published 2019 Sep 10. doi:10.3390/metabo9090180.
-
Wang S, Wan T, Ye M, et al. Nicotinamideriboside attenuates alcohol induced liver injuries via activation of SirT1/PGC-1α/mitochondrial biosynthesis pathway. Redox Biol. 2018;17:89-98. doi:10.1016/j.redox.2018.04.006.
-
Pham TX, Bae M, Kim MB, et al. Nicotinamideriboside, an NAD+ precursor, attenuates the development of liver fibrosis in a diet-induced mouse model of liver fibrosis. BiochimBiophysActaMol Basis Dis. 2019;1865(9):2451-2463. doi:10.1016/j.bbadis.2019.06.009.
-
Han X, Bao X, Lou Q, et al. Nicotinamideriboside exerts protective effect against aging-induced NAFLD-like hepatic dysfunction in mice. PeerJ. 2019;7:e7568. Published 2019 Aug 28. doi:10.7717/peerj.7568.
-
Ralto KM, Rhee EP, Parikh SM. NAD+ homeostasis in renal health and disease. Nat Rev Nephrol. 2020;16(2):99-111. doi:10.1038/s41581-019-0216-6.
-
Hershberger KA, Martin AS, Hirschey MD. Role of NAD+ and mitochondrial sirtuins in cardiac and renal diseases. Nat Rev Nephrol. 2017;13(4):213-225. doi:10.1038/nrneph.2017.5.
-
PoyanMehr A, Parikh SM. PPARγ-Coactivator-1α, Nicotinamide Adenine Dinucleotide and Renal Stress Resistance. Nephron. 2017;137(4):253-255. doi:10.1159/000471895.
-
PoyanMehr A, Tran MT, Ralto KM, et al. De novo NAD+ biosynthetic impairment in acute kidney injury in humans. Nat Med. 2018;24(9):1351-1359. doi:10.1038/s41591-018-0138-z.
-
Zhuo, L., Fu, B., Bai, X., Zhang, B., Wu, L., Cui, J., Cui, S., Wei, R., Chen, X., & Cai, G. (2011). NAD blocks high glucose induced mesangial hypertrophy via activation of the sirtuins-AMPK-mTOR pathway. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology, 27(6), 681–690. https://doi.org/10.1159/000330077
-
Guan, Y., Wang, S. R., Huang, X. Z., Xie, Q. H., Xu, Y. Y., Shang, D., & Hao, C. M. (2017). Nicotinamide Mononucleotide, an NAD+ Precursor, Rescues Age-Associated Susceptibility to AKI in a Sirtuin 1-Dependent Manner. Journal of the American Society of Nephrology : JASN, 28(8), 2337–2352. https://doi.org/10.1681/ASN.2016040385
-
Morigi, M., Perico, L., Rota, C., Longaretti, L., Conti, S., Rottoli, D., Novelli, R., Remuzzi, G., & Benigni, A. (2015). Sirtuin 3-dependent mitochondrial dynamic improvements protect against acute kidney injury. The Journal of clinical investigation, 125(2), 715–726. https://doi.org/10.1172/JCI77632.
-
Tran, M. T., Zsengeller, Z. K., Berg, A. H., Khankin, E. V., Bhasin, M. K., Kim, W., Clish, C. B., Stillman, I. E., Karumanchi, S. A., Rhee, E. P., & Parikh, S. M. (2016). PGC1α drives NAD biosynthesis linking oxidative metabolism to renal protection. Nature, 531(7595), 528–532. https://doi.org/10.1038/nature17184.
Shipping information
- No EU import duties.
- Ships within 1-7 business days.
- Ships in our fully recyclable and biodegradable signature boxes.
Couldn't load pickup availability


NAD+ (Nicotinamide Adenine Dinucleotide)
- Regular price
-
$69.00 USD - Regular price
-
$79.99 USD - Sale price
-
$69.00 USD
Why Choose Us
-
Quality Assurance
Every item is carefully inspected and tested before shipping to ensure it meets our premium quality standards.
-
30-Day Risk-Free Guarantee
If your product arrives damaged, defective, or not as described, we’ll replace it or issue a full refund—no hassle, no stress.
-
Secure Shopping
Your payment and personal information are protected with advanced encryption for a safe and smooth shopping experience.
-
Customer Support You Can Trust
If you ever need help, our support team is ready to assist you with quick, friendly service.

Quick view
#Chillaxin' 3 in 1 Luxury Spray (2 oz.)
-
Regular price
-
$20.00 USD
-
Regular price
-
-
Sale price
-
$20.00 USD
- Regular price
-
$20.00 USD - Regular price
-
- Sale price
-
$20.00 USD
Quick view
#Bookworm Wax Tart
-
Regular price
-
$12.00 USD
-
Regular price
-
-
Sale price
-
$12.00 USD
- Regular price
-
$12.00 USD - Regular price
-
- Sale price
-
$12.00 USD
Quick view
#AfterTheStorm Luxury Candle
-
Regular price
-
$10.00 USD
$33.00 USD
-
Regular price
-
-
Sale price
-
$10.00 USD
$33.00 USD
- Regular price
-
$10.00 USD $33.00 USD - Regular price
-
- Sale price
-
$10.00 USD $33.00 USD



